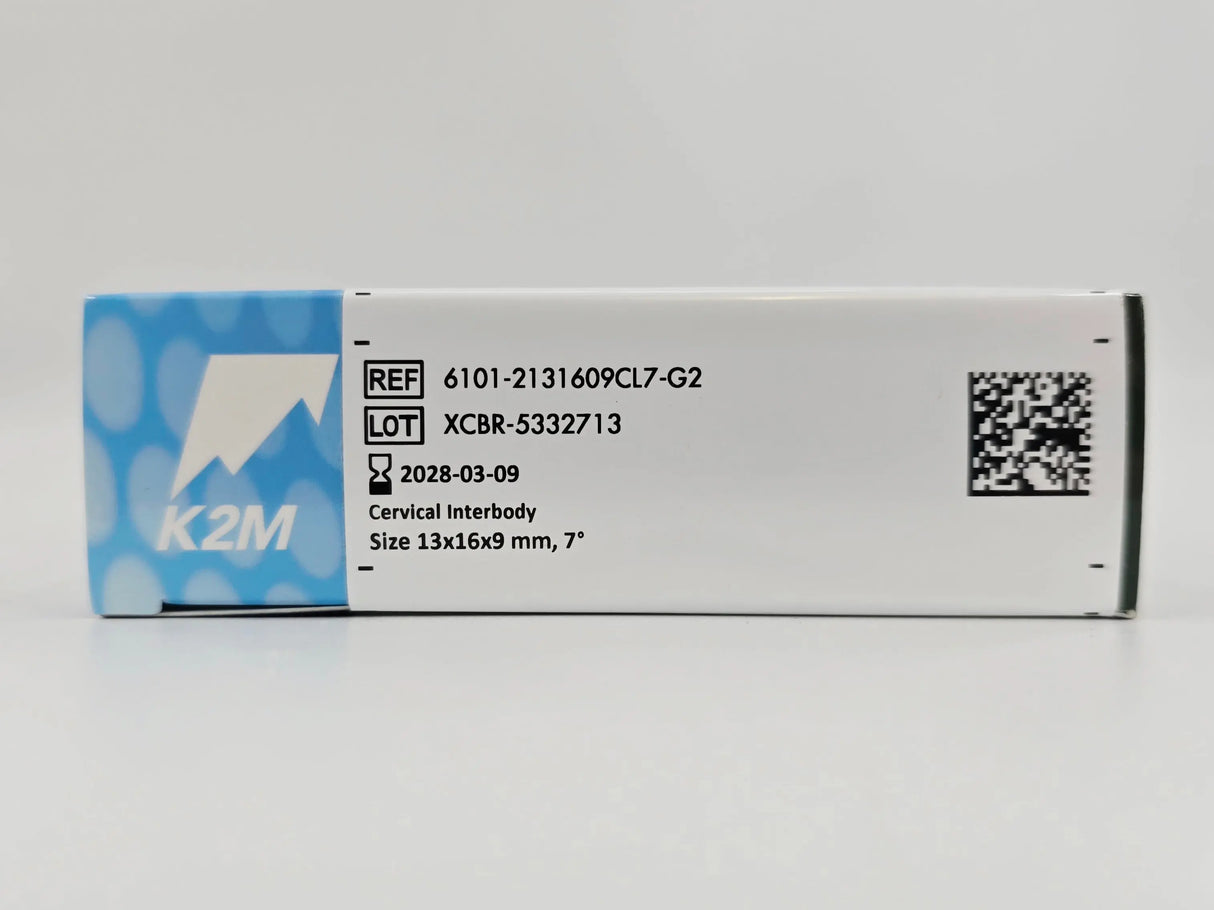
K2M 6101-2131609CL7-G2 Cascadia Interbody System Cervical Interbody 13mm x 16mm x 9mm 7°
K2M 6101-2131609CL7-G2 Cascadia Interbody System Cervical Interbody 13mm x 16mm x 9mm 7° is backordered and will ship as soon as it is back in stock.
Couldn't load pickup availability
K2M 6101-2131609CL7-G2 Cascadia Interbody System Cervical Interbody 13mm x 16mm x 9mm 7°
• Model: 6101-2131609CL7-G2
• Size: 13mm x 16mm x 9mm
• 7°
This medical supply or implant item is securely packaged within its original manufacturer's packaging, which remains undamaged. The inner and outer packaging is intact, ensuring the product's integrity and suitability for medical use.
The CASCADIA Cervical 6101‑2131609CL7‑G2 interbody spacer is a cervical fusion implant designed to be placed between adjacent vertebral bodies in the neck (cervical spine) to restore disc height, support load bearing, and facilitate fusion. Its dimensions (13 mm long × 16 mm wide, with a height of 7 mm) make it appropriate for situations where moderate restoration of disc height is needed without excessive distraction. The device's 7° lordotic angle aids in achieving the desired cervical sagittal alignment, helping maintain or restore natural curvature after disc degeneration or injury.
One of the distinguishing features of this device is K2M’s Lamellar 3D Titanium Technology, which gives the implant a porous, roughened surface structure. Preclinical data suggest such surfaces enhance bone ongrowth and ingrowth compared to smoother or non‑porous metallic implants. The porosity helps create channels and pathways through which bone can penetrate and integrate, potentially improving the rate and strength of fusion. The titanium body provides structural strength, durability, and biocompatibility, with load‑bearing capability immediately post‑implantation—assuming correct placement and supplemental stabilization.
In clinical use, the 13×16×7 mm, 7° variant of the CASCADIA Cervical cage is useful in anterior cervical discectomy and fusion (ACDF) procedures, particularly when surgeons want both moderate lordotic restoration (but not aggressive) and adequate graft surface area. The moderate height helps avoid over‑distraction of neural foramina or excessive stress on endplates, which can reduce risks such as subsidence. The device's well‑designed footprint (width × length) helps distribute load and may reduce point loading. However, selection must carefully match patient anatomy and surgical goals, and success depends on proper endplate preparation, graft choice, and correct orientation. Given its roughened porous design, imaging follow‑up may show different radiopacity or inferential signatures compared to PEEK cages, which might influence radiographic assessment strategies.

No reviews available.
The Primis TeamProudly Supports
-
![]()
DAMIEN HOUSE
Located in Guayaquil, Ecuador, Damien House exists to provide care and help dispel the stigma of those affected by Hansen's Disease (Leprosy).
-
![]()
PROJECT PERFECT WORLD
Project Perfect World provides orthopedic surgery for children worldwide.