MicroAire Carpal Tunnel Release System 81017 w/ Handpiece & 4 Attachments
MicroAire Carpal Tunnel Release System 81017 w/ Handpiece & 4 Attachments is backordered and will ship as soon as it is back in stock.
Couldn't load pickup availability
MicroAire Carpal Tunnel Release System 81017 w/ Handpiece & 4 Attachments
Carpal Tunnel Release System
Included Items:
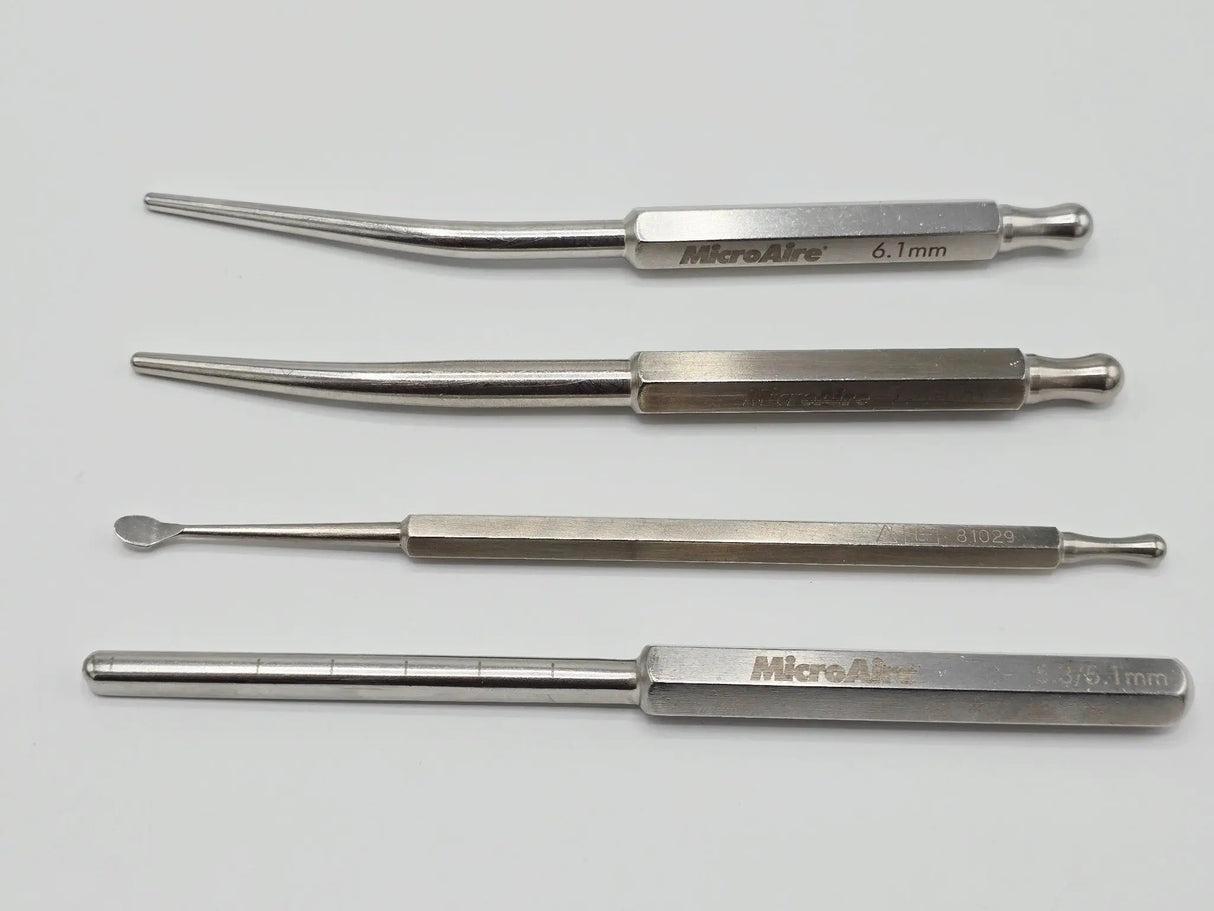
• MicroAire 81017 Carpal Tunnel Release System Case (8" × 7" × 1.5")
• MicroAire 81014 Handpiece
• MicroAire 81026 6.1 mm
• MicroAire 81027 7.4 mm
• MicroAire 81029 Synovium Elevator
• MicroAire 81061 5.3 / 5.1 mm Dual-Sized Cutting Insert
Condition: Used in good condition
MicroAire Carpal Tunnel Release System, including the 81017 sterile case, MicroAire 81014 handpiece, and multiple cutting inserts in various sizes. This system is designed for precise, minimally invasive division of the transverse carpal ligament. MicroAire instruments are known for reliability, durable engineering, and high-performance cutting action used in orthopedic and hand surgery environments.
The set includes five insert sizes plus the powered handpiece, allowing surgeons or training facilities to perform or simulate a wide range of carpal tunnel release procedures. All components fit into the included MicroAire 81017 case for organized storage and transport. Items show normal cosmetic wear consistent with prior use, but all mechanical components appear intact unless otherwise noted.
This system is ideal for training labs, skills courses, research, simulation environments, or veterinary surgical settings. Not sold as sterile. Not for human medical use. Please review all photos to confirm condition and included items.

No reviews available.
The Primis TeamProudly Supports
-
![]()
DAMIEN HOUSE
Located in Guayaquil, Ecuador, Damien House exists to provide care and help dispel the stigma of those affected by Hansen's Disease (Leprosy).
-
![]()
PROJECT PERFECT WORLD
Project Perfect World provides orthopedic surgery for children worldwide.