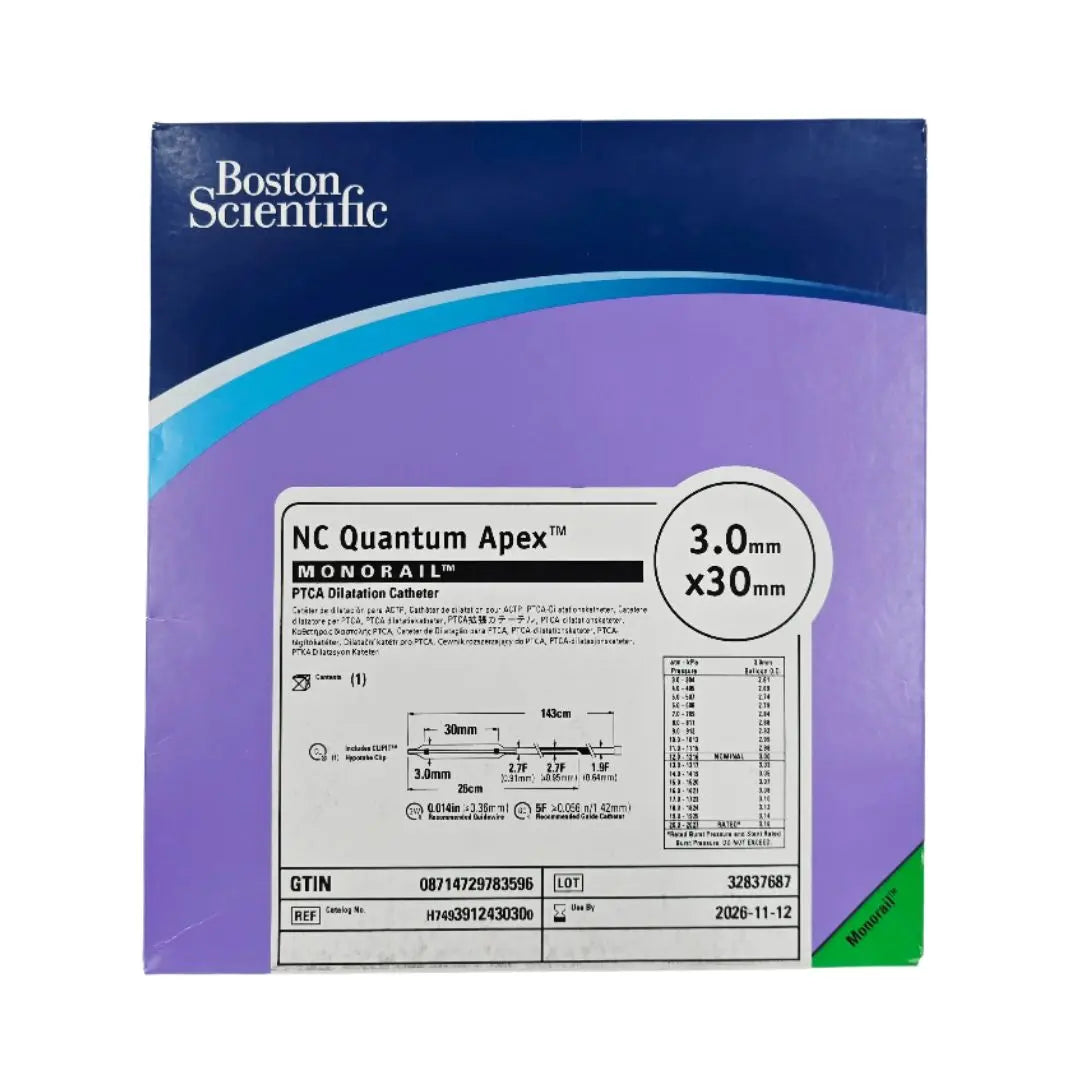
Boston Scientific H7493912430300 NC Quantum Apex Monorail PTCA Dilation Catheter 3.0mm x 30mm
Boston Scientific H7493912430300 NC Quantum Apex Monorail PTCA Dilation Catheter 3.0mm x 30mm is backordered and will ship as soon as it is back in stock.
Couldn't load pickup availability
Boston Scientific H7493912430300 NC Quantum Apex Monorail PTCA Dilation Catheter 3.0mm x 30mm
• Model: H7493912430300
• 3.0mm x 30mm
• Overall Length: 143cm
The NC Quantum Apex 3.0 mm × 30 mm monorail PTCA dilation catheter is a non‑compliant balloon device in the Boston Scientific Quantum Apex line, intended primarily for post-dilatation of coronary stents or dilatation of residual stenoses. With its monorail (rapid exchange) design, it allows the operator to exchange balloon segments with minimal manipulation of guidewire length, improving procedural efficiency especially in coronary settings. A 30 mm balloon length gives it good coverage for longer stent segments or overlapping zones requiring “touch-up.”
One of the key strengths of this catheter is its deliverability: the Quantum Apex family is engineered with enhancements in distal-end flexibility and tip characteristics to reduce “tip catch” and improve recrossing of stent struts. These refinements offer smoother negotiation of tortuous vessels, easier tracking across residual narrowing, and more consistent performance in complex coronary anatomies. The low balloon growth (≈ 4 %) helps ensure that the balloon doesn’t overexpand significantly under high pressure, maintaining controlled dilation and minimizing unintended oversizing of the vessel. The platinum‑iridium markers deliver precise fluoroscopic visibility, aiding in accurate alignment during inflation.
Clinically, operators may choose this 3.0 mm × 30 mm variant when a moderate vessel segment (e.g. mid to large coronary branches) requires post-dilatation over a longer stent length. The 143 cm working length provides suitable reach from standard guide catheters. Because it shares the high burst pressure capability and low overexpansion characteristics of the Apex series, it supports confident “touch-up” dilations after stent deployment. As always, use must be in accord with the device’s indications, with caution against off‑label or high‑risk use (e.g. unprotected left main).

No reviews available.
The Primis TeamProudly Supports
-
![]()
DAMIEN HOUSE
Located in Guayaquil, Ecuador, Damien House exists to provide care and help dispel the stigma of those affected by Hansen's Disease (Leprosy).
-
![]()
PROJECT PERFECT WORLD
Project Perfect World provides orthopedic surgery for children worldwide.