BUSSE 822 IV START KITS - IV Start Kit, Transparent Dressing & Securement, 50/cs (US Only)
BUSSE 822 IV START KITS - IV Start Kit, Transparent Dressing & Securement, 50/cs (US Only) is backordered and will ship as soon as it is back in stock.
Couldn't load pickup availability
BUSSE 822 IV START KITS - IV Start Kit, Transparent Dressing & Securement, 50/cs (US Only)
• Model: 822
• 50 Sterile Kits/ Case
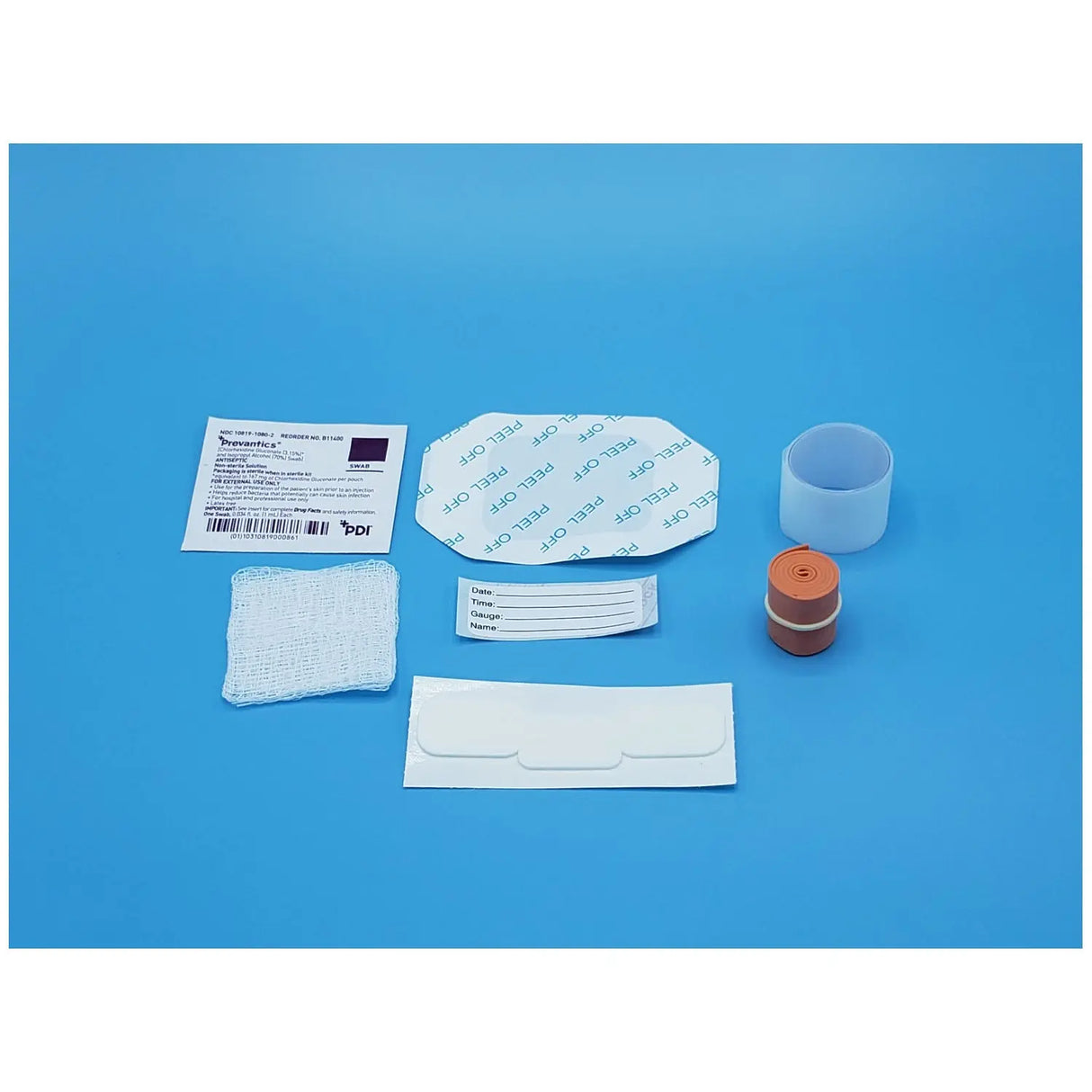
Latex free. Kits contain: (1) tourniquet, (2) 2" x 2" gauze sponges (1) roll tape. Packed in a soft, disposable tray that keeps contents sterile until opened.- IV Start Kit, Transparent Dressing, Chlorascrub Swab, Posi-Guard Catheter Securement Device, Sterile, 50 kt/cs (US Only)
The Busse IV Start Kit #822 is a comprehensive, ready‑to‑use kit developed for healthcare professionals to streamline the process of establishing and securing peripheral intravenous (IV) access. Each kit arrives in a sterile soft pack tray that keeps everything organized, allowing clinicians to focus on the procedure rather than assembling supplies. The inclusion of a transparent window dressing ensures optimal visualization of the insertion site, while the Posi‑Guard catheter securement device helps stabilize the IV line and reduce line movement or dislodgement once inserted.
What distinguishes this kit is its integration of essential components for each step of the IV start process—from skin antisepsis (via a ChloraScrub swab) to site dressing, securement, labeling and documentation. Because all items are included and packaged for single use, the kit reduces the risk of missing supplies, cross‑contamination, or protocol deviations. The latex‑free tourniquet contributes to a safer environment for patients with latex sensitivities, and the sterile packaging aligns with infection‑control best practices in acute care, outpatient and inpatient settings.
From an operational standpoint, supplying standardized kits like the Busse #822 helps streamline inventory management and procedural consistency across units. Having the same kit used by multiple clinicians reduces variation, thereby supporting best‑practice adherence and potentially reducing complication rates associated with IV insertions (such as dislodgement or infiltration). Because it is packaged in cases of 50 kits, it remains cost‑effective for larger facilities and supports continuous availability for busy IV teams.
No reviews available.
The Primis TeamProudly Supports
-
![]()
DAMIEN HOUSE
Located in Guayaquil, Ecuador, Damien House exists to provide care and help dispel the stigma of those affected by Hansen's Disease (Leprosy).
-
![]()
PROJECT PERFECT WORLD
Project Perfect World provides orthopedic surgery for children worldwide.